Electrolytes Ratio
Written by Ben Bunting: BA, PGCert. (Sport & Exercise Nutrition) // British Army Physical Training Instructor // S&C Coach.
--
Electrolytes are chemicals that conduct electricity when dissolved in liquids.
Electrolytes play an essential role in maintaining nerve and muscle function, hydrating our bodies, balancing blood acidity and pressure levels, healing tissue damage and helping the immune system fight infection.
Common examples include sodium, potassium, calcium, magnesium and phosphate.
Under an electric charge, chemical elements in a solution may naturally separate into positive and negative ions, with positive ones known as cations possessing more electrons than negative anions (known as anions).
As soon as they're pushed together by an electric field they jump across solution boundaries between electrodes to form an electrical current requiring electrolytes as conductors of this movement of ions and ultimately create an electrical current requiring their conductance through electrolytes.
Human bodies contain several fluid compartments containing electrolytes, with their concentration determining how much water moves into or out of that compartment through an osmotic process known as osmosis.
Extracellular fluid contains sodium as its key electrolyte; its concentration can control how much water moves in or out via osmosis.
Other electrolytes include chloride, magnesium and phosphate in extracellular fluid - also lungs and kidneys are responsible for regulating bicarbonate (HCO3) concentration to help buffer acid produced during normal metabolic processes produced as byproducts of metabolism.
Electrolyte levels in your body may fluctuate for many reasons, including loss of fluid during exercise and diarrheal illness as well as hormone secretion.
Diet and supplements may help manage low levels of certain electrolytes.
Electrolytes Outlined
Electrolytes, also referred to as electrically active substances (electrolytes), are chemical substances that conduct electricity when dissolved in water.
They're essential components of our bodies for various reasons such as regulating nerve and muscle function, hydrating the body, helping balance blood acidity/pressure balance and rebuilding damaged tissue.
Your body gets its daily intake from food and fluid sources; any excess electrolytes are excreted via urine.
The primary electrolytes include sodium, potassium, calcium, magnesium, phosphorus and bicarbonate.
Our kidneys use these electrolytes to keep concentrations of these electrolytes relatively constant regardless of other changes to body functions.
Nervous and muscular cells (nerve and muscle tissue) use them to maintain voltage across their cell membranes and carry electrical impulses (nerve impulses or heartbeat signals) between themselves and other cells.
Chemical Elements
Electrolytes contain chemical elements with either positive or negative electrical charges, which when dissolved in water balance each other out so the solution easily conducts electricity.
A good example is salt water: its sodium (positively charged) and chlorine (negatively charged) atoms move around, without disturbing neutral molecules of water, creating what is called ionization.
Strong electrolytes like salt do so completely while weak ones (like non-salts) tend to only partially ionize (typically 1-10%).
If you experience symptoms of electrolyte imbalance, speaking to a health care professional is key for understanding if it's just an electrolyte imbalance or more serious medical conditions that need to be addressed first.
A metabolic panel laboratory test may also look into your kidney function and blood sugar levels to provide accurate feedback.
Sodium
Extracellular fluid is a mixture of electrolytes that includes sodium, a cation with osmotically-active cation.
It regulates the membrane potential and maintains the extracellular fluid.
Active transport involves the exchange of sodium and potassium across cell membranes.
The kidneys regulate sodium. Most sodium reabsorption occurs in the proximal tube.
The sodium is reabsorbed in the distal convoluted tube. The sodium transport is controlled by aldosterone via sodium-chloride synporters.
Hyponatremia, the most common electrolyte disorder, is the most common.
When the serum sodium concentration is below 135 mmol/L, hyponatremia can be diagnosed. Neurological manifestations of hyponatremia are common.
Patients can present with headaches and confusion. They may also experience nausea or delirium. Hypernatremia is when the serum sodium level exceeds 145 mmol/l.
Hypernatremia can cause restlessness, sleep difficulty and tachypnea.
The rapid correction of sodium can cause severe complications such as cerebral edema or osmotic demyelination (ODS).
ODS can also be caused by other factors, such as chronic alcohol abuse disorder or malnutrition.
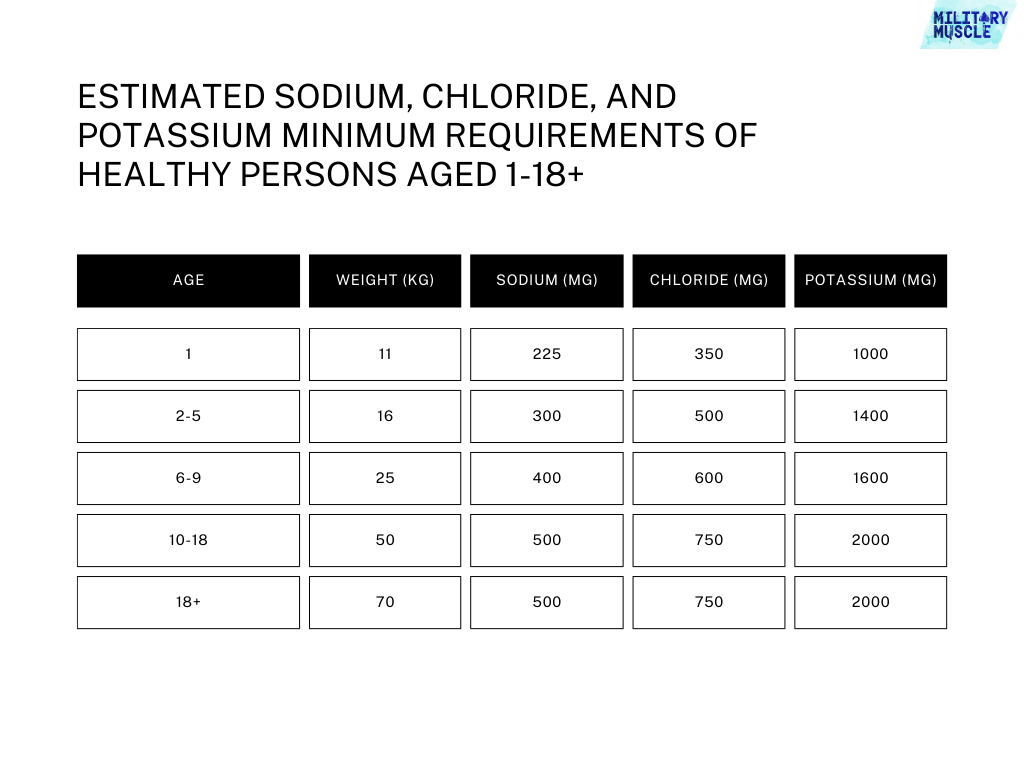
The amount required to support growth is dependent on the rate of expansion of extracellular fluid, which varies according to age and reproductive status.
General Requirements
Adults In a climate with moderate temperatures, a healthy adult can maintain a sodium balance by consuming very little sodium.
In the 1950, it was estimated that the daily urinary and fecal excretion of adults is 23 mg (1mEq).
Sweat is another source of sodium loss. It has a normal average of 25 mEq/liter
British men and woman lost only 2 to 5 % of their sodium through sweat and fecal excretion.
The amount of dermal loss that is required to be taken into account has been estimated to range between 46 and 92 mg (2-4 mEq).
In conditions of maximum adaptation, without active sweating, a daily average of 5 mEq for adults is estimated. This corresponds to 115mg of sodium, or about 300mg of sodium chloride.
Potassium
Potassium is mostly an intracellular ion.
The sodium-potassium triphosphatase pumps is responsible for the regulation of the homeostasis in sodium and potassium. It exchanges sodium for potassium which enters the cells.
The glomerulus is the site where potassium is filtered in the kidneys.
The thick ascending Henle loop and the convoluted tubule at its proximal end are responsible for potassium reabsorption.
The distal convoluted tubeule is where potassium secretion takes place. Aldosterone increases the secretion of potassium.
Potassium is also secreted by potassium channels and potassium chloride cotransporters located at the apical membrane of tubular cells.
Arrhythmias can be caused by potassium derangements. Hypokalemia is when the serum potassium level falls below 3.6 mmol/L.
Hypokalemia is characterized by weakness, fatigue and muscle twitching. Hypokalemic Paralysis is a generalized weakness of the body that can either be familial or sporadic.
Hyperkalemia is when serum potassium levels exceed 5.5 mmol/L. This can lead to arrhythmias.
Hyperkalemia can be diagnosed by signs and symptoms such as muscle cramps, weakness, rhabdomyolysis and myoglobinuria.
General Requirements
Only a few studies have evaluated the Potassium needs of adults.
While the losses from a diet low in potassium or "minimum potassium" are minimal, potassium is not as well conserved as sodium.
The feces are not more than 400mg (10mEq). Renal losses can be 200-400mg (5-10mEq).
Other losses (e.g. in sweat) are negligible. With intakes up to about 20 mEq/day metabolic balance can be achieved, but at the cost of lower potassium levels in the body (upto 250 mEq), and sometimes even plasma levels.
For a normal body store and normal concentration of plasma and interstitial liquid, an intake up to 40 mEq/day is needed.
It would seem that the minimum daily requirement is between 1,600 and 2,000 mg (40-50 mEq).
It is well-established that potassium in the diet has a positive effect on hypertension. Increased intake of fruits, vegetables and legumes would increase potassium intake for adults to approximately 3,500 mg (90mEq) each day.
Chloride
The extracellular fluid is a major source of chloride. Serum chloride is primarily regulated by the kidneys.
The majority of chloride, which is filtered out by the glomerulus (mostly by the distal tubule), is reabsorbed both by active and passive transport by both proximal as well as distal tubules.
Hyperchloremia can occur due to gastrointestinal bicarbonate loss.
Hypochloremia can manifest as gastrointestinal symptoms like nausea or excessive water retention like congestive cardiac failure.
A chloride deficiency in the diet is very rare. In Western diets, the main source of dietary chlorine is sodium chloride. This salt is added to food during industrial processing or for discretionary purposes.
Chloride can also be found in food and food additives that contain chloride. In these cases, chloride is associated with other cations than sodium.
In healthy individuals, chloride is absorbed efficiently in the gastrointestinal tract. After absorption, the chloride anions travel freely in the blood where they are maintained at a specific concentration.
General Requirements
A chloride deficiency in the diet is very rare. In Western diets, the main source of dietary chlorine is sodium chloride. This salt is added to food during industrial processing or for discretionary purposes.
Chloride can also be found in food and food additives that contain chloride. In these cases, chloride is associated with other cations than sodium.
In healthy individuals, chloride is absorbed efficiently in the gastrointestinal tract. After absorption, the chloride anions travel freely in the blood where they are maintained at a specific concentration.
Reference values for sodium can be equal to those for chloride for all populations groups. They are:
- Children aged 3 to 5 years: 1.7 grams per day
- Children aged 6 to 12 years: 2 g/day
- Children aged 7-10: 2.6 g/day
- Children aged 11-17 Years: 3.1 grams per day
- Adults, including lactating and pregnant women, should consume 3.1 grams of calcium per day.
These levels of chloride intake, which are consistent with the reference value for sodium, are considered safe and adequate for general populations.
Magnesium
Magnesium is a cation that occurs intracellularly.
Magnesium plays a major role in adenosine Triphosphate (ATP), muscle function, neurotransmitter production, and neurological function. Magnesium is responsible for calcium reuptake in the sarcoplasmic membrane by the calcium activated ATPase.
When the serum magnesium level is less than 1,46 mg/dL, it is called hypomagnesemia.
Hypomagnesemia can be caused by alcohol use disorder, gastrointestinal disorders, and excessive renal losses.
Torades de Pointes and ventricular arrhythmias are common.
Certain medications such as omeprazole can also cause hypomagnesemia.
Magnesium is found in approximately 25 grams of magnesium in an adult's body. Between 50 and 60% of this mineral can be found in bones, while the remainder is in soft tissue.
Blood serum contains less than 1% total magnesium, and the levels are closely monitored.
The normal serum magnesium concentration ranges between 0.75 and 0.9 millimoles/L.
A serum magnesium level of less than 0.75mmol/L is considered hypomagnesemia.
Magnesium is controlled primarily by the kidney. It excretes approximately 120 mg of magnesium per day into the urine.
Magnesium deficiency can reduce urinary excretion.
General Requirements
For adults aged 19-51 years, the Recommended Dietary Amount (RDA) is 400-420mg daily for men & 310-320mg for women.
Lactation requires 310-320mg daily, while pregnancy needs 350-360mg.
Calcium
Calcium plays a vital role in the human body. Calcium is important for skeletal mineralization and contraction of muscles.
It also plays a role in nerve transmission, blood clotting and hormone secretion.
Calcium is primarily obtained from food.
Calcium is an extracellular cation.
The 1,25-dihydroxy form of vitamin D3 is the most active hormonal form that controls calcium absorption.
The distal tubules of the kidneys are also regulated by parathyroid hormone. Calcitonin decreases calcium levels in blood by acting on bone cells.
General Requirements
Calcium and vitamin D are both essential for bone building, as we know from studies.
You should get enough calcium if you consume at least 700mg of it through food.
If you are unsure, consult your doctor first before taking a calcium-based supplement.
Even though vitamin D is a natural source, it can be difficult to get enough from the sun and diet.
Many doctors recommend vitamin D supplements containing between 800 and 1,000 IU per day.
Phosphorous
Each cell of our body contains phosphorus. The majority of phosphorus can be found in the teeth and bones, but some is also in your genes.
You need phosphorus in your body to produce energy and carry out important chemical reactions.
The extracellular fluid cation of phosphorus is hydroxyapatite. Eighty-five per cent of total body phosphorus resides in bones and teeth as hydroxyapatite. The soft tissues contains the remaining 15 per cent.
The phosphorus plays an important role in metabolic pathways. It is a constituent of many metabolic intermediaries and, most important, of ATP, and nucleotides.
Vitamin D3, PTH and calcitonin regulate calcium along with phosphate. The kidneys are the main route of phosphorus elimination.
Most often, the cause of phosphate imbalance is one of three things: poor dietary intake, gastrointestinal problems, or abnormal renal excretion.
General Requirements
The Recommended Daily Allowance (RDA), for adults men and women aged 19 years or older, is 700mg per day.
For those lactating and pregnant require 700 mg of phosphorus daily.
Bi-carbonate
Bicarbonate levels are determined by the acid-base balance of blood.
The kidneys are primarily responsible for maintaining the acid-base equilibrium and regulating bicarbonate.
The kidneys reabsorb filtered bicarbonate, and produce new bicarbonate through net acid excretion. This occurs when titrable acids and ammonia are excreted.
In most cases, diarrhea results in a loss of bicarbonate. This imbalance can lead to an acid-base disturbance.
Bicarbonate excess can be caused by kidney disorders and imbalanced metabolism.
General Amounts for Use
Standard doses of sodium-bicarbonate supplementation in athletes (200-300mg/kg) are proven to improve performance, especially when the performance is heavily affected by metabolic acidosis.
Electrolyte Ratio's
In one study, it was found that few adults meet the recommended intake ratios of sodium to potassium (1.2% vs. 1.5%), sodium to calcium (12.8% vs. 17.67%) and sodium and magnesium (13.7% vs. 7.3%).
About half of all adults (55.2 % of men and 54.8 % of women) meet the calcium-magnesium ratio recommendation.
It is common to blame sodium for raising blood pressure, while potassium is credited with keeping it under control.
These two minerals work together in the body, so it's not a good idea to separate them.
It is more important to consider the ratio of potassium and sodium in your diet than either amount alone.
The Paleolithic hunter/gatherers consumed about 11,000 mg (milligrams) of potassium per day, mainly from vegetables, fruits, flowers, leaves and roots. They ate less than 700 mg sodium.
This is a ratio of sodium to potassium of 1:16. We get more potassium (2,500mg) than sodium (3400mg), a ratio of 1.36:1.
If you have high blood tension, increasing your potassium intake may help lower it.
By lowering your blood pressure, potassium can reduce the risk of heart disease and stroke. Consuming too much sodium, on the other hand, can increase your blood pressure.
If you suffer from high blood pressure (also known as hypertension), it is important to limit your sodium intake.
Heart disease and stroke are linked to high blood pressure.
The majority of potassium in our diet comes from fruits, vegetables, dairy, and seafood.
The majority of sodium in our diet is found in packaged foods and restaurant food.
What is the proper ratio of sodium potassium and magnesium?
According to research, the correct electrolyte ratio is:
- 1000 milligrams sodium
- 200 milligrams potassium
- 60 micrograms magnesium
The electrolytes in your body are essential for all your cells, but especially your neurons or nerve cells.
Even small reductions in electrolyte levels or dehydration may lead to cognitive and physical impairment.
This is especially important after a heavy sweating session, like during or after an exercise or sauna.
Hydration and Electrolytes
The average adult body contains 60% water. This means that nearly all fluids and cells in your body contain electrolytes.
Electrolytes help regulate chemical reactions and maintain fluid balance inside and outside of your cells. Electrolytes are obtained by your body from food and drinks.
The amount of water that you need to drink each day can vary depending on a variety of factors. These include the temperature, the physical activity level, the body composition, the age and fluid intake.
Drinks account for approximately 80% daily water consumption and are therefore considered the primary way to maintain hydration.
It has been established that, in addition to water, different beverages can provide fluids to the body.
Salt can be added to fluids, for example, and this will improve fluid retention. This is especially true when electrolyte losses are high due to sweating caused by exercise.
In addition, athletes who consume other macronutrients, such as carbohydrates and protein, benefit from a fluid balance that is restored, in addition to the recovery of exercise due to the replenishment of muscle glycogen.
The maintenance of optimal hydration is of interest to the general public and does not necessarily relate to rehydration after exercise.
Water content in beverages is absorbed into the body's water pool by a number of physiological processes including intestinal absorption and gastric emptying. It then escapes through various channels, most notably urine (in absence of sweating).
Electrolyte Imbalance Treatment
Treatment for electrolyte balance depends on the electrolytes that are out of equilibrium, whether there is too much or not enough, and what's causing it.
In mild cases, it may be enough to change your diet. Other cases may require other treatments. You can, for example:
- Electrolyte Replacement Therapy is used if you do not have enough electrolyte. It involves giving you more electrolyte. You can take a pill or drink a supplement, or you can get it intravenously.
- Your doctor will give you fluids or medicines (by IV or mouth) to help you remove electrolytes from your body if you have too many. You may require dialysis in severe cases to remove the electrolyte.
Conclusion
The correct electrolyte ratio, according to science, is 1000 mg of sodium, 200 mg of potassium and 60 mg of magnesium.
These electrolytes play a vital role in the health of your cells, especially your neurons and nerve cells.


